

 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. People | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A member of the First Year Chemistry team will be available to answer questions in The Concept Room in the Chemistry Resource Centre. If you have any questions, please come see us, we would be more than happy to assist you. If you have an urgent matter that must be dealt with immediately, please contact the First-Year Chemistry Coordinator. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Schedule | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chem 1012 is a 3
credit hour course. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
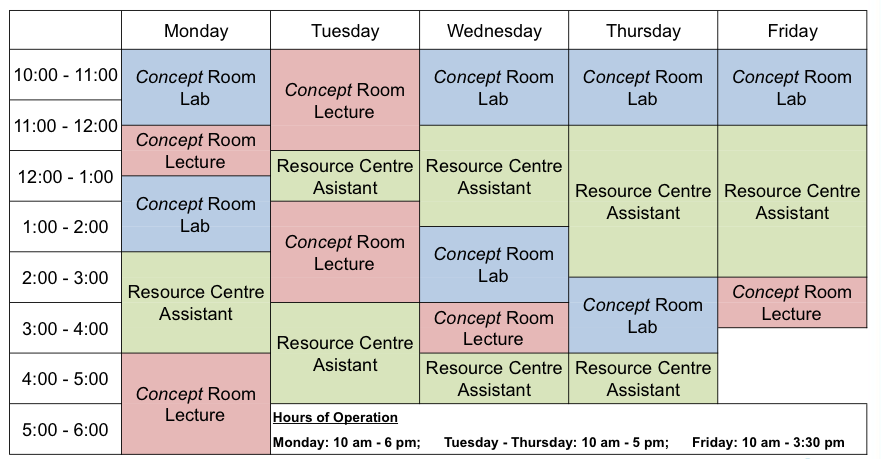
Lectures: Lecture times are shown in the following table: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Laboratory Schedule: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Laboratories | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| To
pass Chem 1012, the laboratory must also be passed (7.5/15).
Your Lab Instructor will give further information concerning the running of the laboratories. The Lab Instructors are responsible for making all decisions concerning the running and grading of the laboratories. Queries concerning the laboratories should be directed to them. We have made every effort to ensure that the laboratories occur after you have met the relevant material in class. Nevertheless, it is simply not possible to arrange every laboratory in such a manner. In those instances where the laboratories precede the classroom material, we have attempted to make the laboratory serve as an introduction to those particular classes. Hence, the lab manual contains detailed background sections designed to give you enough information to comprehend the aim of the experiment. These sections should all be read carefully, since many offer a different perspective on the material. Missed Labs Lab Exemptions Lab Coats As
part of your
chemistry laboratory class requirement, all students who did not take
Chem 1011 in the Fall of 2014 must complete the chemistry safety
module. This must be
completed by 11:30
pm January 18, 2015. Students who do not
successfully complete this requirement will not be allowed to perform
experiments in any Dalhousie undergraduate chemistry laboratory.
Successful completion of the Safety Module includes reading the General
Safety Statement in your lab manual, obtaining a perfect mark (e.g.,
10/10 or 12/12) on five online quizzes, and completing the lab map
portion during your first lab time. After completion of these
requirements you should feel comfortable working in a chemistry
laboratory and have the tools you need to promote a safe laboratory
environment. The Safety Module can be found on the Bblearn site: CHEM - Laboratory Safety
Module - 2015 Winter Prelab
Exercises You must first
register for CAPA before you can access these prelab exercises.
Please refer to Section 7 of this document for instructions on how to
register for CAPA. We have done our best to ensure that the prelab questions are free from errors. However, if you do not agree with an answer or feel that you have not received the grade that you deserve, you can apply (in writing) for a grade adjustment to a prelab exercise. To apply for a grade adjustment for a prelab question, select "Send Message" at the bottom of the page for the specific CAPA pre-lab question and explain why you feel your grade should be adjusted. Make sure the "Question about resource content" option is selected before sending your message. Lab
Protocol |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Textbook | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. The Resource Centre | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The Chemistry Resource Centre is located on the first floor of the Chemistry building. The Resource Centre is staffed by advanced undergraduate students in Chemistry during the times that there is not a member of the first year team in The Concept Room. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The Chemistry Resource Centre will open Tuesday, January 6th at 10:00 AM.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6. The Concept Room | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Informal scheduled tutorials will be held by members of the first year team in The Concept Room. The Concept Room is located inside the Chemistry Resource Centre. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The Concept Room will open Tuesday, January 6th
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lecture Support | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
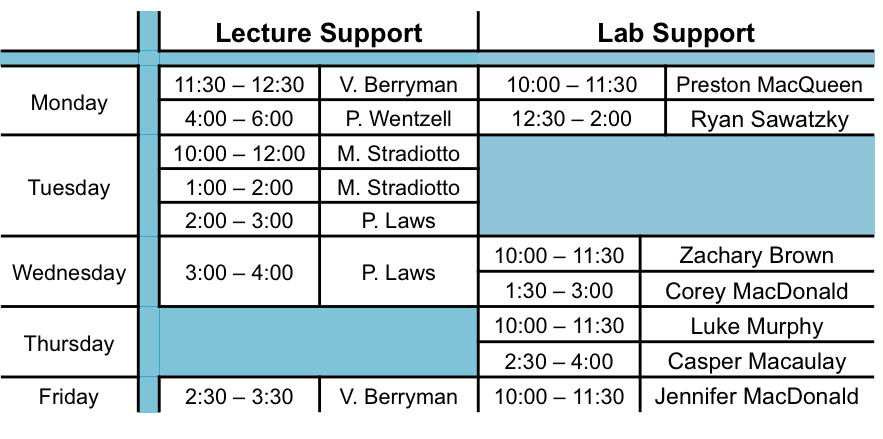
We encourage you to come to The Concept Room for help to further your understanding of the material covered during class as well as help with the assignments. Additional tutorials will be scheduled prior to the term tests and final exam. Notices will be posted on Bblearn before the tutorial. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lab Support | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| We encourage you to come to The Concept Room to further your understanding of the material covered in lab as well as help with the lab reports. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7. CAPA Assignments | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Registering for the online CAPA assignments :
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Accessing the online CAPA assignments :
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Assignments Throughout the term you will be required to complete 10 on-line assignments. You will be assigned a term-mark based on your best 9 results. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The online assignments are comprised of "practice assignments" and "graded assignments." | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Practice
Assignments: Graded
Assignments: We encourage you to refer to the Assignment Guide for tips on inputting your answers to the assignment questions. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The deadline for each assignment will be strictly adhered to, no exceptions. Consequently, make sure that you start early enough to enable you to get help with the questions that cause you difficulty. All assignments are available now. The due dates for the assignments are summarized in the following table: |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Applications
for Grade Adjustments: To apply for a grade adjustment for an assignment question, select "Send Message" at the bottom of the page for the specific CAPA question and explain why you feel your grade should be adjusted. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Assignment DO’s and DON’Ts: DO start your assignment early. DO go to The Concept Room and The Resource Centre for help with assignment questions and class material. DO discuss your assignments with your colleagues. We encourage you to help each other further your understanding of the material. DO use your textbook to help answer the questions. DON’T do someone’s assignment for them or let them do it for you (this don’t is also regulated by the rules for Academic Honesty!). The only way to develop your understanding of Chemistry is to work through the material yourself. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8. Tests | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
There will be TWO tests given during the term. Test 1:This test will be given on Friday, January 30, 2015 from 7:00 - 8:00 pm. You will be allowed to bring one handwritten 8.5 x 11 piece of paper (single-sided, containing the necessary formulas and any other pertinent information). You will be allowed to bring a single-line entry calculator. Use of programmable calculators or any other electronic device will not be allowed during the test. You will be provided with the periodic table and constants sheet (available on BbLearn site). Test 2: This test will be given on Friday, March 7, 2015 from 7:00 - 8:30 pm. You will be allowed to bring one handwritten 8.5 x 11 piece of paper (single sided, containing the necessary formulas and any other pertinent information) and a single-line entry calculator to the test. Only single line entry calculators will be allowed. Use of programmable calculators or any other electronic device will not be allowed during the test. You will be provided with the periodic table and constants sheet (available in BbLearn). Missed Tests If you miss the test, you MUST contact Patricia Laws within 24 hours of the missed test for further instructions . If appropriate documents (such as a medical certificate) are submitted to the First Year Chemistry Coordinator (Patricia Laws) in a timely manner, the missing term test will be replaced by the final exam mark.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
IMPORTANT TEST DATES
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9. Examinations and Grading Scheme | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chem 1012 Final Exam | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| All
sections of 1012 will write a common final examination during the April
examination period. The final examination will be 3 hours in
duration and will include all material covered during the term (see
syllabus below). The exam schedule is set by the Registrar's Office
and is usually available about 6 weeks into the term. Students who are ill or unable to make the final exam must contact the first-year coordinator (Patricia Laws) within 24 hours of the missed exam for further instructions. In order to be allowed to write a make-up exam, students must provide the appropriate documentation (i.e. medical excuse) to the First Year coordinator within 1 week of the regularly scheduled exam. You will be allowed to bring one handwritten 8.5 x 11 piece of paper (double sided, containing the necessary formulas and any other pertinent information) and a calculator to the final exam. Only single line entry calculators will be allowed. Use of programmable calculators or any other electronic device will not be allowed during the exam. You will be provided with the periodic table and constants. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chem 1012 Grading Scheme |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| If it is necessary to change this scheme for any reason, you will be informed by your professor in class and on the Bblearn class site. If changes have to be made they will apply equally to all sections of CHEM 1012. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Important Notes
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1. In order to obtain a passing grade in Chem 1012, you must meet all of the following criteria:
Students who do not meet these criteria will not receive a passing grade in Chem 1012. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Grades | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
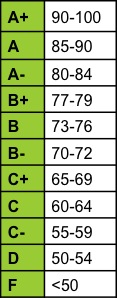
| Numerical grades will be converted to letter grades according to the following scale: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
2.
Under
emergency circumstances that have a serious impact on the delivery of
this class, there may be a need to alter the syllabus. It is each
student's responsibility to notify the first year coordinators
(Patricia Laws, Patricia.Laws@dal.ca; Jennifer MacDonald,
jennifer.l.macdonald@dal.ca) regarding absences due to illness. For
missed
tests Patricia Laws must be contacted within 24 hours of the missed
test or exam. For missed labs, please refer to the policy in the lab
manual and contact Jennifer MacDonald. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10. Faculty of Science Academic Integrity
Module and Policy |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| As
part
of the class requirement, you are encouraged to complete the Faculty of
Science Academic Integrity Module regardless of faculty or year of
study if you have not yet completed it. Successful completion of this module involves reading the
Academic Integrity module and completing the Academic Integrity quiz
and
Academic Integrity agreement. The academic integrity module is located on the BbLearn site entitled "Writing Centre Academic Integrity Module." |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
INTELLECTUAL
HONESTY
Department of Chemistry, Dalhousie University
A university should be a model of intellectual honesty. Failure to meet the University’s standards in this regard can result in an academic offence. The length of time a student has attended university, the presence of a dishonest intent and other circumstances may all be relevant to the seriousness with which the matter is viewed. Violations of intellectual honesty are offensive to the entire academic community, not just to the individual faculty member and students in whose class an offence occurs. Instructors are responsible for setting examinations and assignments as part of the learning process and for evaluating those examinations and assignments, including ensuring that any rules stated for the procedures used in an examination or assignment are followed. Any violation of such stated rules which could result in a student gaining advantage may be considered to be an academic offence. Examples of Academic Offences Plagiarism
The University attaches great importance to the contribution
of original thought to learning and scholarship. It attaches
equal importance to the appropriate acknowledgement of sources from
which facts and opinions have been obtained. The proper use of
footnotes and other methods of acknowledgement vary from one field of
study to another. Failure to cite sources as required in the
particular field of study in the preparation of essays, term papers and
dissertations or theses may, in some cases, be considered to be
plagiarism. Students who are in any doubt about how to
acknowledge sources should discuss the matter in advance with the
faculty members for whom they are preparing assignments. In many
academic departments, written statements on matters of this kind are
made available as a matter of routine or can be obtained on
request. Students may also take advantage of resources available
through the Writing Centre at writingcentre.dal.ca or the Dalhousie
Libraries at infolit.library.dal.ca/tutorials/plagiarism/. Irregularities in the
Presentation of Data from the Experiments, Field Studies, etc. Other Irregularities
Aiding in the Commission of
an Academic Offence
Misrepresentation
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11. Student Services | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Student Services offers a number of programs to support academic advancement as well as personal and professional development. The services are outlined on their website: www.studentservices.dal.ca | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Student Accessiblity Services | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Students
may request accommodation as a result of barriers related to
disability, religious obligation, or any characteristic under the Nova
Scotia Human Rights Act. Students who require academic accommodation
for either classroom participation or the writing of tests and exams
should make their request to the Advising and Access Services Center
(AASC) prior to or at the outset of the regular academic year. Please
visit www.dal.ca/access for more information and to obtain the Request for Accommodation -- Form A. A note taker may be required as part of a student's accommodation. There is an honorarium of $75/course/term (with some exceptions). If you are interested, please contact AASC at 494-2836 for more information. Please note that your classroom may contain specialized accessible furniture and equipment. It is important that these items remain in the classroom, untouched, so that students who require their usage will be able to participate in the class. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Studying for
Success |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The
transition from high school to university life can often be a
challenging one. However, with help from the Studying for Success
program, you too can become a more effective learner. Attend our
workshops or drop in for individual study skills sessions, where we can
help you with Time Management, Critical Reading, Note taking, Preparing
for Exams, and much more. Don’t wait until it’s too late! Let Studying
for Success help you find smarter ways to study. For more information or to make appointments, please: - Visit our website: www.dal.ca/sfs - Visit our main office in the Killam Library, Room G28 (main floor) - Call 494-3077 or - Email the Coordinator at: sfs@dal.ca |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12. Chem 1012 Syllabus | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| What follows is a description of the syllabus for Chem 1012. This and any other material covered will be examined in your assignments, term tests and the final examination. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Objectives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Our objectives for Chem 1012 are to offer you a comprehensive and relevant course on the fundamental concepts in chemistry. We have literally written the book for you to ensure that you have chemistry knowledge to proceed into second year courses. The Department of Chemistry is striving to ensure that you receive a coordinated effort with hand grading by faculty members and the opportunity to get extra help from a First Year Chemistry Professor in The Concept Room (located in the Chemistry Resource Centre) or by downloading one of our Chemistry Online Video Tutorials (located on Bblearn). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Topics | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||